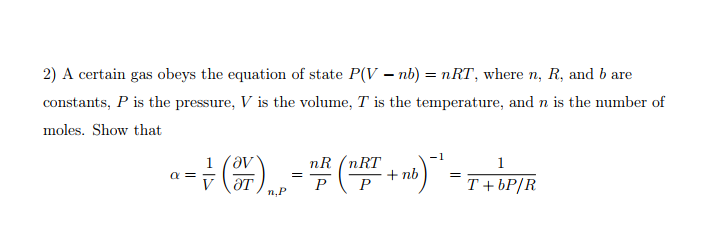
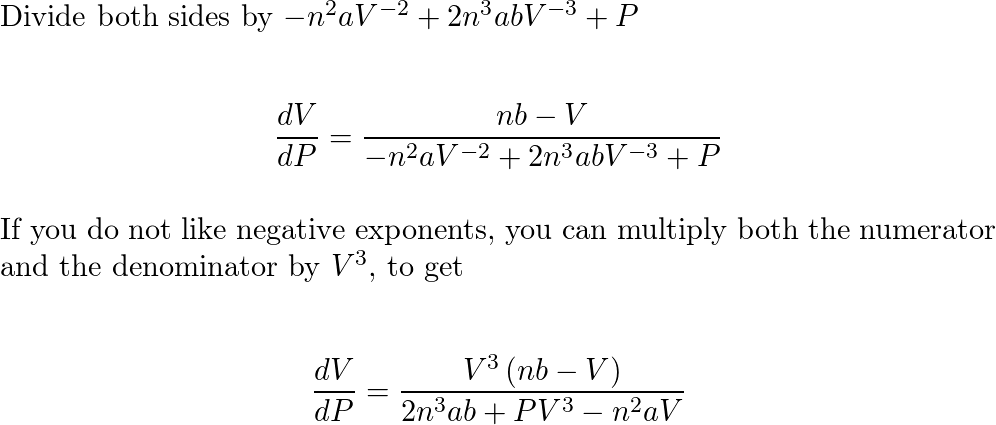The equation of state of n moles of a non - ideal gas can be approximated by the equation (P + an^2V^2)(V - nb) = nRT where a and b are constants

A good coilover kit for an excellent price. Fixed damping matched to the springs. Exclusive to FM! – flyinmiata
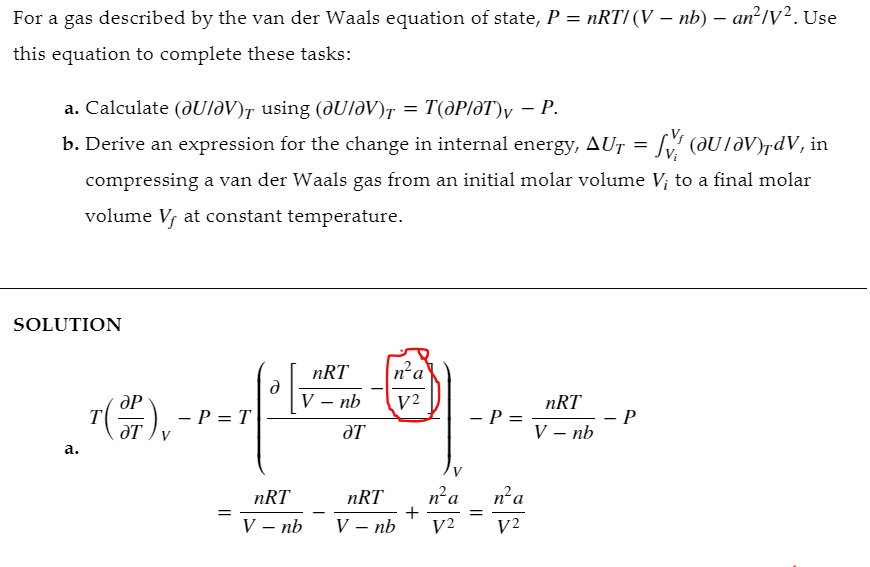
SOLVED: For a gas described by the van der Waals equation of state, P = nRTI (V nb) an?/V? . Use this equation to complete these tasks: Calculate (JUIdV)r using (JUIIV)T T(PIdT)v -
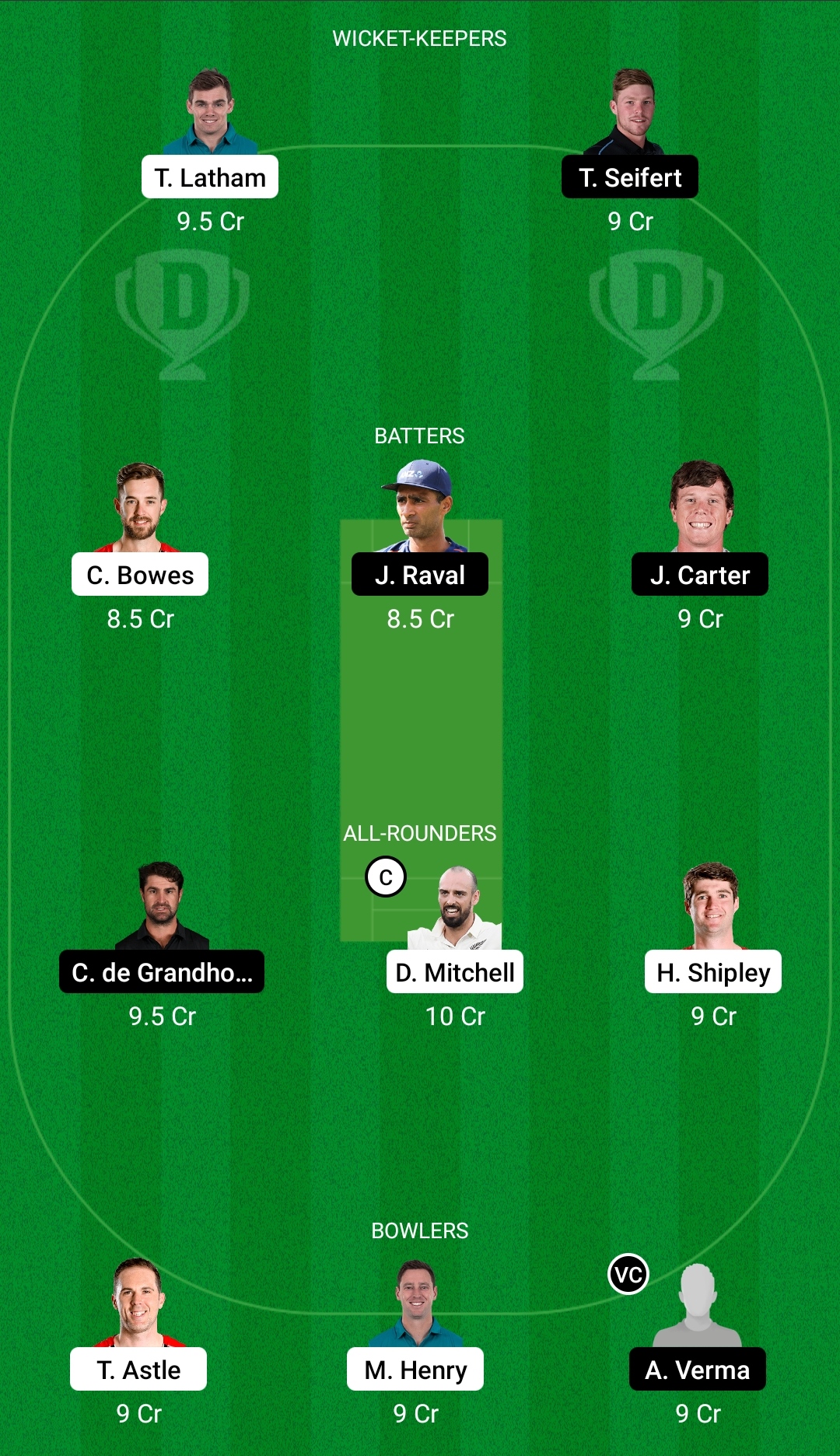
CTB vs NB Dream11 Prediction, Fantasy Cricket Tips, Dream11 Team, Playing XI, Pitch Report, Injury Update- Dream11 Super Smash T20
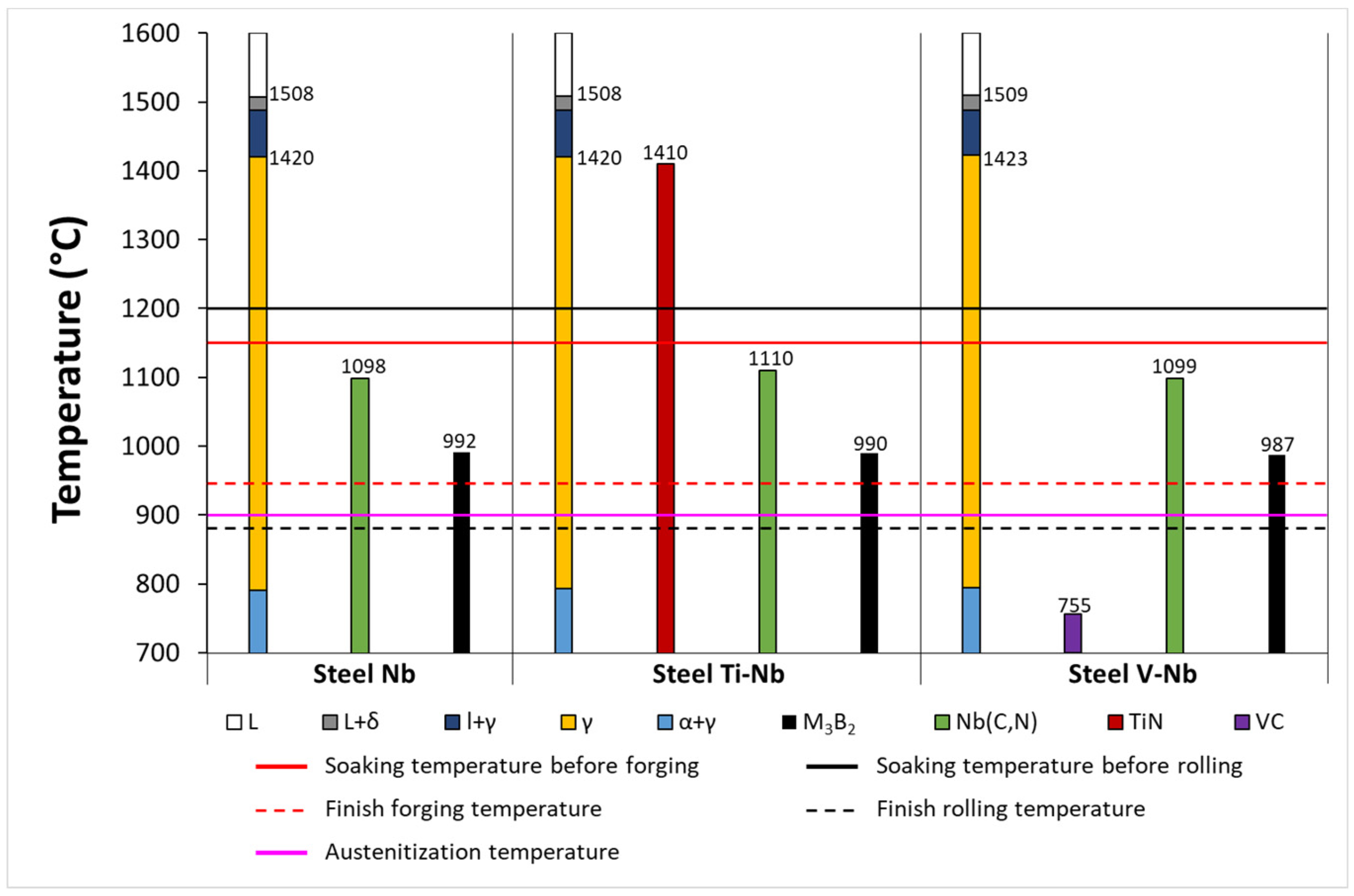
Metals | Free Full-Text | Grain Size Evolution and Mechanical Properties of Nb, V–Nb, and Ti–Nb Boron Type S1100QL Steels

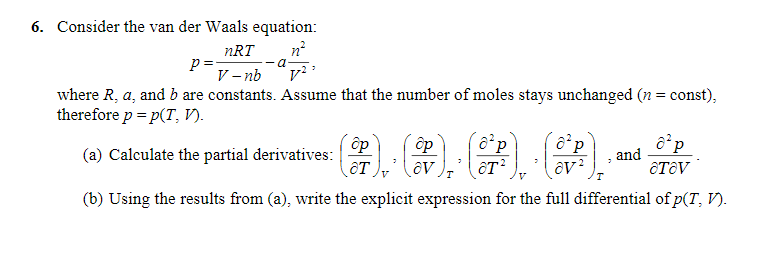
SOLVED:(a) The van van der Waals equation for n moles of a gas is (P + (n^2 a)/(V^2))(V - nb) = nRT where P is the pressure, V is the volume, and